We, Dept of Molecular Tumor Biology, study the mechanisms of development and progression of malignant tumors. Our main themes can be broadly divided into the following three categories;
- Study of molecular mechanisms of osteosarcoma development and pregression.
- Study of molecular mechanisms of tongue cancer progression.
- Discovery of novel anti-tumor drugs derived from natural compounds.
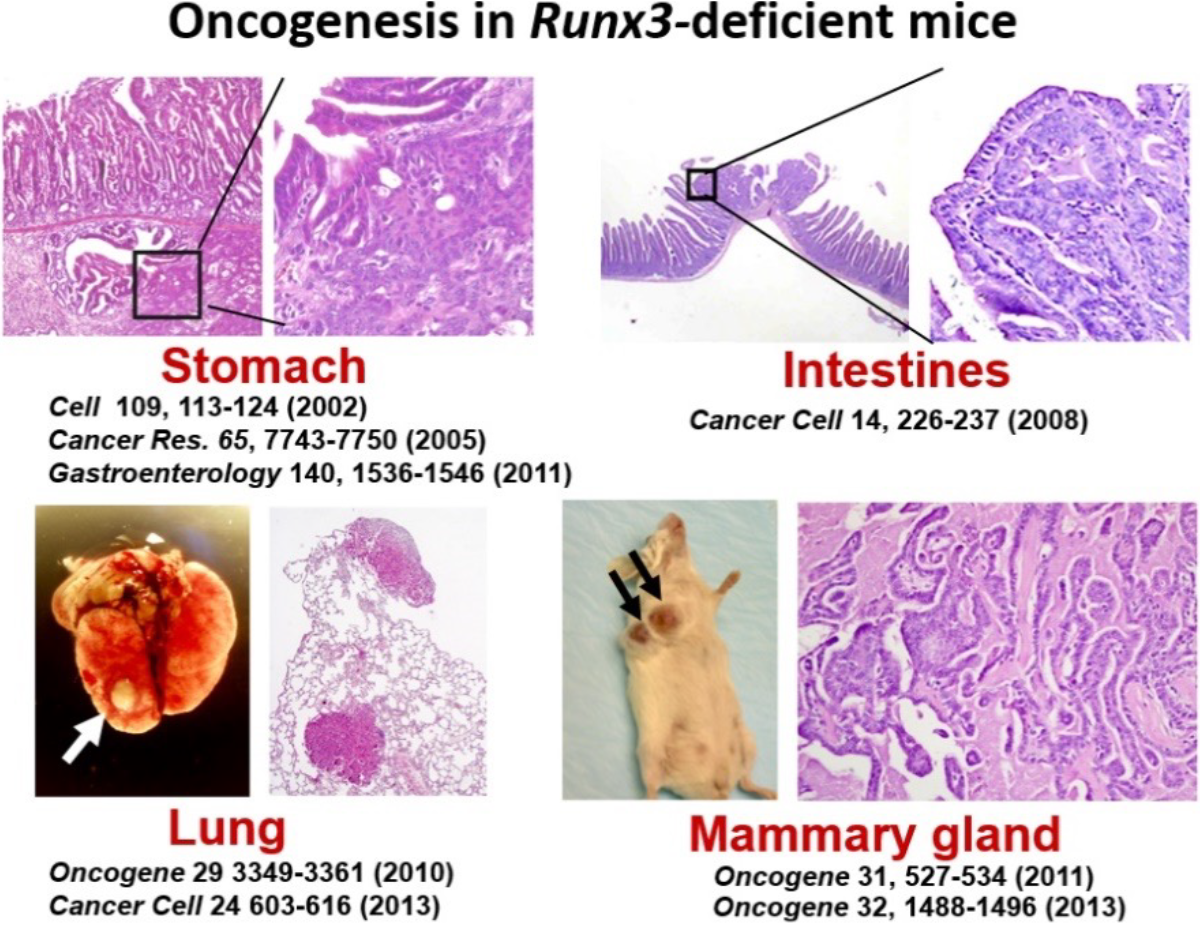
We have consistently promoted functional analysis of the transcription factors RUNXs, and RUNX family genes (RUNX1, 2, and 3) are known to function as "oncogenes" or "tumor suppressor genes". In particular, the functional involvement of RUNX3 has been reported in almost all human cancers, since we were the first in the world to generate Runx3 knockout mice and to clarify the causal relationship between Runx3 and gastrointestinal cancers (Cell, 109, 113-124, 2002; Cancer Res, 140, 1536-1546, 2011; Cancer Cell, 14, 226-237, 2008; Gastroenterology, 138, 255-265, 2010; Gastroenterology, 140, 1536-1546, 2011; Oncogene 31, 527-534, 2012).
Runx3-deficient mice develop not only gastrointestinal cancers but lung and breast cancers at a high frequency.
Recently, our analysis of osteosarcoma (and T-cell lymphoma) has revealed that Runx family genes strongly upregulate the most potent "oncogene," Myc, under conditions in which p53, the most universal "tumor suppressor gene" in human cancer, is inactivated (Oncogene 41, 683-691, 2022; Oncogene 42, 2485–2494, 2023; Cancer Science 115, 2961–2971, 2024; Gene 819, 146234, 2022). Thus, in the absence of p53, the RUNX transcription factor is thought to function as an oncogene.
We are now developing mouse models to analyze the malignant mechanism of not only osteosarcoma but "tongue cancer," the most important malignant tumor in the field of dentistry.
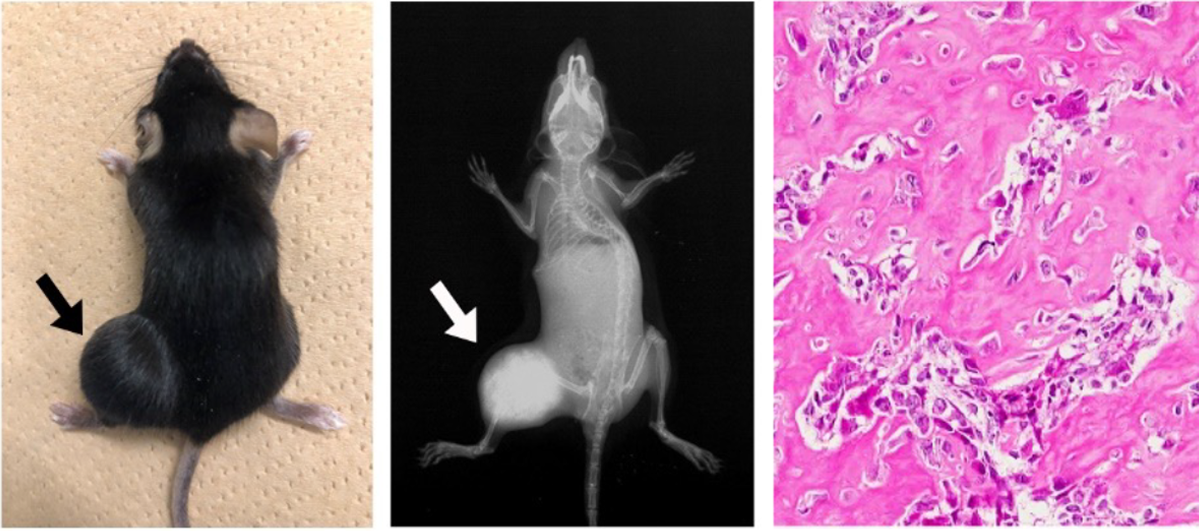
Osteoprogenitor-specific p53 deletion causes osteosarcoma, which closely resembles human osteosarcoma in nature. In this osteosarcoma, Runx3 is highly upregulated, accompanied by excessive Myc.
RUNX, which functions as an oncogene only in the absence of p53, is very attractive as an anti-tumor target. Therefore, we are searching for effective and specific inhibitors of RUNX using
Nagasaki University Marine Microbial Extract (NU-MME) library 
. We aim to identify anti-tumor agents derived from natural compounds with minimal side effects and to apply them to clinical practice.